Health
Understanding COVID-19 vaccine hesitancy among blood cancer patients
2022
PARTNERS
Leukaemia & Lymphoma Society
Share
In collaboration with The Leukaemia & Lymphoma Society, we surveyed behavioural barriers to vaccination among 6,500 blood cancer patients.
Is the vaccine safe for individuals who have been diagnosed with cancer? Professional health associations recommend cancer patients to get vaccinated.1,2 However, some individuals are understandably concerned. Evidence suggests that cancer patients are more vulnerable to the disease, 3, 4 which naturally raises additional questions about vaccine safety and effectiveness in this specific group.
Understanding and addressing specific concerns of blood cancer patients can make them more willing to listen to, and follow, professional guidance on vaccination. Partnering with The Leukaemia & Lymphoma Society, we conducted the largest survey of U.S. cancer patients to date to better understand their attitudes, beliefs and behaviours related to COVID-19.
Our survey showed that one in five blood cancer patients hesitates to get vaccinated
About 70% of blood cancer patients living in the U.S. reported being very likely or likely to get vaccinated if the vaccine was free and readily available. Approximately 13% of blood cancer patients were undecided and 17% were unlikely or very unlikely to get vaccinated.
Compared to vaccine-acceptant patients, the hesitant group perceived a lower risk of contracting COVID-19 and a lower risk of getting hospitalised if they contracted the disease. Vaccine-hesitant patients also tended to be younger, non-white, female and more likely resided in a rural or suburban area.
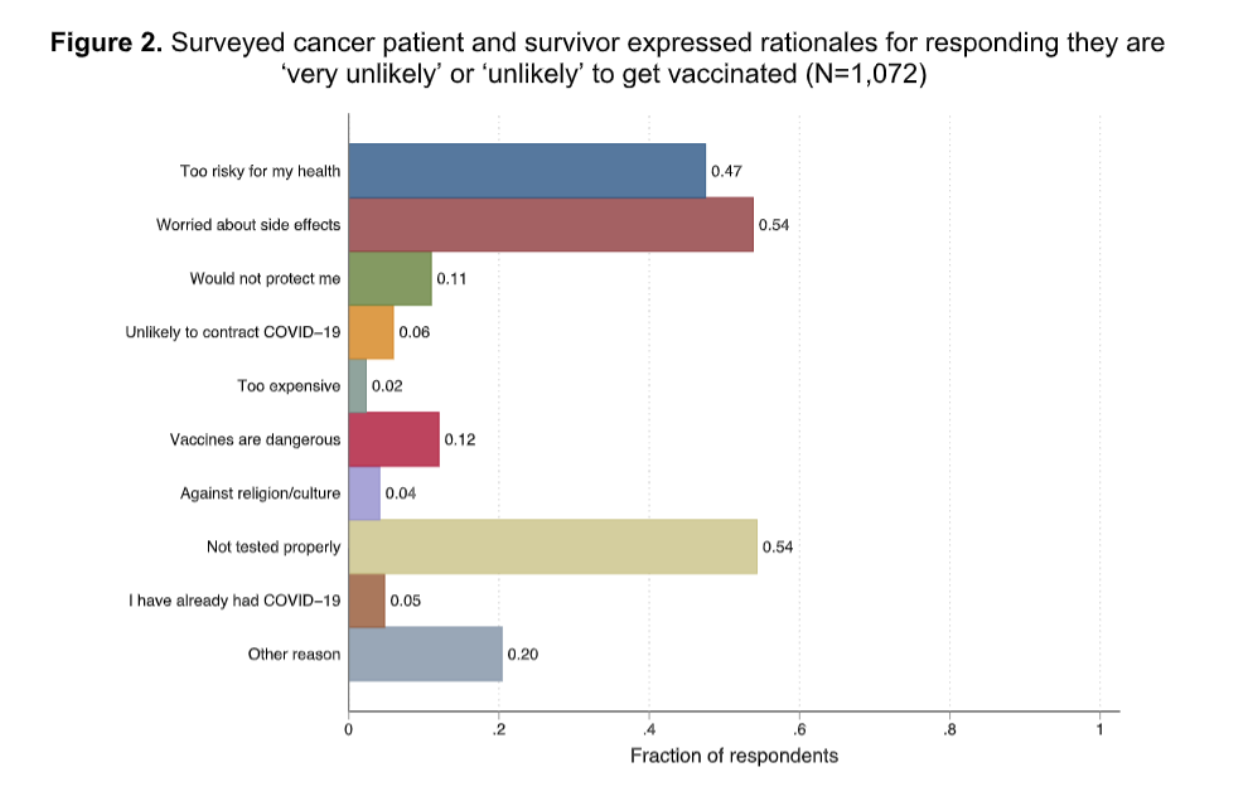
Top reasons for vaccine hesitancy: lack of adequate testing and worry about side effects
The majority of hesitant respondents were concerned about vaccine safety. As cancer patients were excluded from vaccine testing trials in the US, it was not surprising to learn that some cancer patients perceived the vaccine as insufficiently tested and potentially having negative side effects. With the vaccine being rolled out and more granular data becoming available, it is imperative to communicate new evidence to cancer patients so they develop trust in the vaccine.
Besides safety concerns, some respondents questioned the vaccine effectiveness. Given that cancer patients tend to have a lower immune response compared with the general public, 1 the effectiveness of the vaccine in blood cancer patients might be different than in the general population. The public health authorities should consider this and be prepared to answer patient queries about whether the vaccine will protect them
Public and health officials must address group-specific concerns to successfully tackle the pandemic
Clear and consistent messages are the backbone of effective communications. To overcome vaccine hesitancy, these principles must be paired with understanding of concerns that develop in diverse communities. Community surveys can provide quick insight into vaccine-related concerns and effectively inform the communications of public health authorities and professional associations. When trying to get more community members vaccinated, listening to group-specific concerns and using the best available evidence to address these is a key path forward.
- National Comprehensive Cancer Network. Preliminary Recommendations of the NCCN COVID-19 Vaccination Advisory Committee.; 2021. Accessed April 15, 2021. https://www.nccn.org/covid-19/pdf/COVID- 19_Vaccination_Guidance_V1.0.pdf
- American Society of Clinical Oncology. COVID-19 Vaccine Patients with Cancer.; 2021. Accessed April 15, 2021. https://www.asco.org/asco- coronavirus-resources/covid-19-patient-care-information/covid-19-vaccine-patients-cancer
- Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020;383(26):2586-2588.
- Bange EM, Han NA, Wileyto P, et al. CD8 T cells compensate for impaired humoral immunity in COVID-19 patients with hematologic cancer. Res Sq. Published online February 2, 2021. doi:10.21203/rs.3.rs-162289/v1